In simple terms, aging is defined as the process of becoming older, which involves a number of biological mechanisms that lead to deterioration of health – both cognitive and physical – over time.
Of course, aging is inevitable. While many of us would like to stop the clock and avoid blowing out those birthday candles – an unsubtle reminder that we are another year older – it is beyond the realms of medical science.
What may be within reach one day, however, are ways to reduce or reverse the effects of aging, and we’re not talking about anti-aging face creams or cosmetic surgery.
Increasingly, studies have focused on strategies that could combat aging at its core – the cellular processes that contribute to age-related diseases and changes in our physical appearance as we become older.
In this spotlight, we explore the biological causes of aging, investigate what strategies researchers are proposing to fight the effects of aging, and look at what you can do to boost your chances of healthy aging.
Many researchers believe the effects of aging are a result of numerous genetic and environmental factors, and these effects vary from person to person.
The genetic aging theory suggests that, just like hair color and height, our lifespan is influenced by the genes we inherit from our parents.
Such a theory may ring true; studies have shown that children of parents who have a long lifespan are more likely to live a longer life themselves.
And research from Sweden’s Karolinska Institutet (resource no longer available at www.nature.com) – published in 2013 – suggested that the aging process is influenced by mitochondrial DNA that we inherit from our mothers.
The team found that female mouse models passed mutations in mitochondrial DNA – which they accumulated through environmental exposures during their lifetime – to offspring, which reduced their lifespan.
But while evidence for the genetic aging theory is strong, the fact remains that healthy aging and longevity is largely influenced by our environment – that is, what we eat, how much we exercise, where we live and the compounds and toxins we are exposed to throughout our lifetime.
Our DNA accumulates damage from environmental exposures as we age. While cells are capable of repairing most of this damage, sometimes it is beyond repair.
This most often occurs as a result of oxidative stress, where the body does not possess enough antioxidants to fix the damage caused by free radicals – uncharged molecules that cause DNA damage. Oxidative stress has been identified as a key player in the aging process.
Another major cause of DNA damage is the shortening of telomeres. These are the caps at the end of each DNA strand that protect our chromosomes – the thread-like structures that contain all our genetic data.
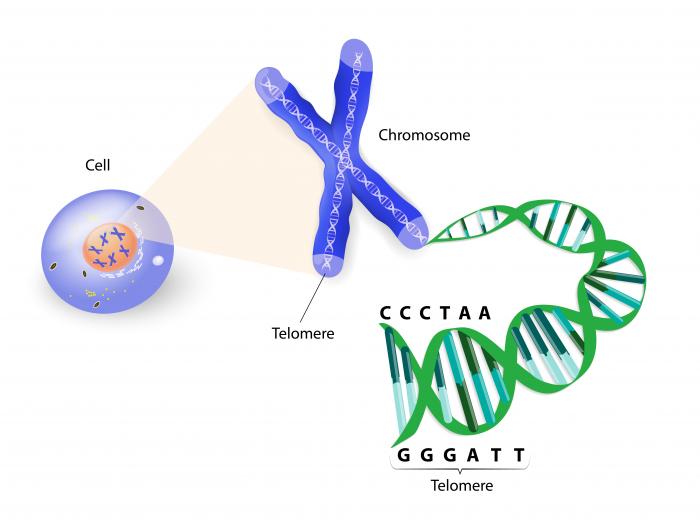
Telomeres are the caps at the end of each DNA strand that protect our chromosomes; their shortening speeds up the aging process.
Telomeres naturally shorten as we age, reducing in length each time a cell divides. But when telomeres become too short, they are no longer able to protect the chromosomes, leaving them susceptible to damage that can lead to premature aging and disease development.
A recent study from the UK’s University of Cambridge suggests that telomere shortening as a result of environmental exposures may even be passed to offspring.
The team found that rats that had lower oxygen in the womb during pregnancy – often caused by smoking during pregnancy in humans – gave birth to offspring with shorter telomeres than rats that had higher oxygen exposure.
What is more, the oxygen-deprived offspring were found to have abnormalities in their blood vessels – a sign of faster aging and a predisposition to heart disease.
“We already know that our genes interact with environmental risk factors, such as smoking, obesity and lack of exercise to increase our risk of heart disease,” notes senior author Prof. Dino Giussani, from the Department of Physiology Development & Neuroscience at Cambridge, “but here we’ve shown that the environment we’re exposed to in the womb may be just as, if not more, important in programming a risk of adult-onset cardiovascular disease.”
The evidence for telomere length as a major player in the aging process has become so strong that researchers are looking to use telomeres as a biomarker for age-related diseases.
Last year, for example, Medical News Today reported on a study in which researchers revealed how a distinct telomere pattern in the blood could be used to predict cancer development.
But what if researchers found a way to extend telomere length to protect against age-related diseases and the other effects of aging? Or what if they identified a strategy that could protect against oxidative stress?
Such approaches may not be too far from reality.
Source: www.medicalnewstoday.com




